Abstract
Introduction: Available treatments for multiple myeloma (MM) have improved rapidly in recent years. However, due to huge disparities in economy, healthcare infrastructure, and accessibility to new drugs, there are big barriers to provide optimal treatment for MM patients in Asian countries. Until now, there are few data on treatment patterns for MM in real practice setting of Asian countries. In terms of continuous improvement of MM management, it is necessary to ascertain the treatment patterns for achieving optimal treatment. Therefore, we investigated the actual treatment patterns and outcomes of MM patients in Korea using the National Health Insurance Service (NHIS) database.
Methods: Newly diagnosed MM patients were identified from the Korean NHIS database between 1st January 2010 to 31st December 2018. Patients had diagnosis with MM (ICD-10 codes C90.0) confirmed with rare and intractable disease registration program. Patients that received at least one stem cell transplantation (SCT) were classified into SCT patients and those without SCT to non-SCT patients. SCT patients and non SCT patients were divided into before and after 1st October 2015 (reimbursement date of bortezomib/dexamethasone [Vd], bortezomib/thalidomide/dexamethasone [VTd] regimens), and 1st December 2017 (reimbursement date of lenalidomide/dexamethasone [Rd] regimen) respectively.
MM treatment was defined to start from the first prescription claim including MM treatment following the diagnosis index date, and the therapies within 14-days period from the first date of each line of therapy (LOT) were considered to constitute the treatment regimen of the respective LOT. When a new MM therapy that was not part of the prior LOT appeared in the prescription claim, LOT was considered to advance to the next LOT. Treatment duration, and treatment-free interval times between LOTs were estimated up to 5th LOT.
Median overall survival (OS) was assessed by Kaplan Meier. Comparison between groups was evaluated by Log rank and Wilcoxon test.
Results: The study population included total of 9,986 newly diagnosed MM patients, 7,501 non-SCT and 2,485 SCT for baseline analyses. Incidence of MM gradually increased by 5.7% of an average annual percent change, from 841 in 2010 to 1,309 in 2018. The mean age of the non-SCT group was older than the SCT group (71.6±9.4 vs 56.7±6.7). The proportion of patients aged <65 was 36.9% and ≥65 was 63.1%. Regardless of SCT eligibility (<65 years), 24.9% of MM patients received SCT. Of the patient eligible for SCT, 62.0% received SCT.
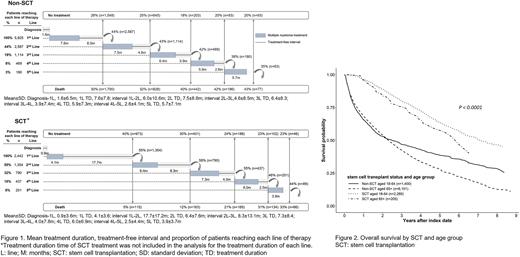
Those 8,367 patients (non-SCT:5,925 / SCT:2,442) with at least ≥1 prescription claims with MM treatment after index date were included for treatment pattern analyses. The proportion of patients advancing to each subsequent LOT was higher across all LOT for SCT patients (range, 44%-58%) than non-SCT patients (range, 35%-44%) and decreased with each line. Higher proportion of patients remained to receive 5th LOT in SCT patients than non-SCT (8% vs 3%). Treatment duration and treatment-free interval between LOTs decreased as the LOTs advanced in both SCT and non-SCT patients. Treatment-free interval was the longest after the 1st LOT in SCT patients which was 17.7 months (Fig. 1). The most common 1st LOT treatment regimen for the non-SCT patients was bortezomib/melphalan/prednisolone and for the SCT patients, thalidomide/dexamethasone was mostly used for induction therapy in the 1st LOT. This trend changed as new regimens were available by reimbursement in the 1st LOT: for SCT patients, to VTd; and for non-SCT patients, to Rd.
The median OS of all patients was 3.36 years (95% CI: 3.22-3.50). The median OS of the SCT patients was significantly longer with median OS of 7.04 years (95% CI: 6.49-8.11) compared to 2.32 years (95% CI: 2.22-2.44) for the non-SCT patients (p<0.0001). When stratified by age and SCT, SCT patients of younger age (< 65 years) had the longest median OS of 7.10 years (95% CI: 6.56-8.20) (Fig. 2). OS was significantly improved for the SCT group after the reimbursement of Vd and VTd in the 1st LOT (p=0.0019).
Conclusions: This real-world data suggests that MM treatment evolves rapidly following the reimbursement of new novel drugs, to treat patients with the most optimal treatment available. And these results show that the OS improves as new novel drugs are incorporated to the treatment. Also, SCT remains an essential treatment providing OS benefit.
Disclosures
Lee:Janssen Korea: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal